
The carbon atom in the center is sp3 hybridized. There are four sigma bonds between C and H. In the CH4 lewis structure, there is a single bond between carbon and hydrogen atoms.Extremely combustible gas and is utilized to generate energy.As there are four bonding pairs of electrons, they adopt the tetrahedral molecular shape to minimize their repulsive forces. According to the Valence shell electron pair repulsion (VSEPR) theory, the same kind of electron pair repels each other. There are four covalent bonds between hydrogen atoms and central carbon atoms in the methane molecule. (c) E° for M 2+(aq) (where M = Ca, Sr or Ba) is nearly constant.Methane has a tetrahedral molecular geometry with bond angles of 109.5°. (b) Lithium is the only alkali metal to form a nitride directly.

(a) The mobilities of the alkali metal ions in aqueous solution are Li + < Na + < K + < Rb + < Cs + (b) Toluene, p-H 3C-C 6H 4-NO 2, p-O 2N-C 6H 4-NO 2.Ĭomment on each of the following observations: (a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene What are the necessary conditions for any system to be aromatic?Īrrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E + Calculateģ4.05 mL of phosphorus vapour weighs 0.0625 g at 546 ☌ and 0.1 bar pressure. A volume of 10.0 L (measured at STP) of this welding gas is found to weigh 11.6 g. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. Enthalpy of formation of CH 4(g) will beĪ welding fuel gas contains carbon and hydrogen only. The enthalpy of combustion of methane, graphite and dihydrogen at 298 K are, –890.3 kJ mol –1, –393.5 kJ mol –1, and –285.8 kJ mol –1 respectively. While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Recently Viewed Questions of Class 11 Chemistry Which one of the following will have largest number of atoms?ĭensity of a gas is found to be 5.46 g/dm 3 at 27 ☌ at 2 bar pressure. (iii) 100 atoms of A + 100 molecules of B In a reaction A + B2 → AB2 Identify the limiting reagent, if any, in the following reaction mixtures. What is the change in internal energy for the process?Īt 0☌, the density of a certain oxide of a gas at 2 bar is same as that of dinitrogen at 5 bar. In a process, 701 J of heat is absorbed by a system and 394 J ofwork is done by the system.

What will be the minimum pressure required to compress 500 dm 3 of air at 1 bar to 200 dm 3 at 30☌? (c) H 2O 2 (aq) + Fe 2+ (aq) → Fe 3+ (aq) + H 2O (l) (in acidic solution) (b) MnO 4 – (aq) + SO 2 (g) → Mn 2+ (aq) + HSO 4 – (aq) (in acidic solution) (a) MnO 4 – (aq) + I – (aq) → MnO 2 (s) + I 2(s) (in basic medium)
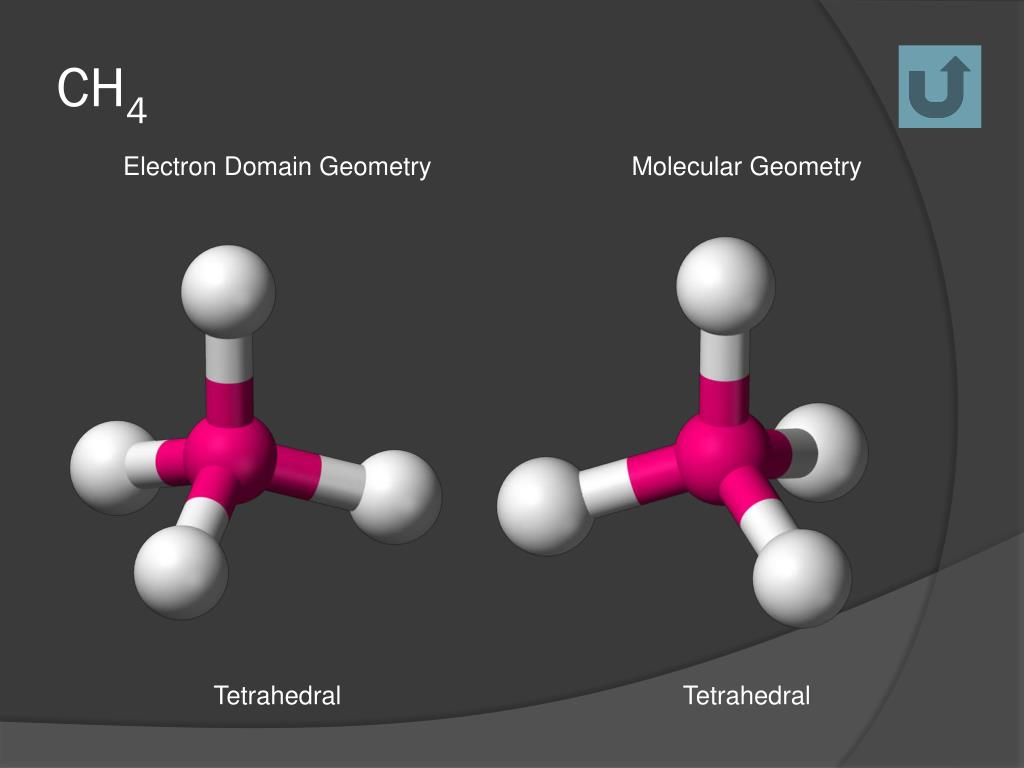
is 3.0 × 10 –25 J, calculate its wavelength.Ĭalculate the wavelength of an electron moving with a velocity of 2.05 × 10 7 ms –1.īalance the following redox reactions by ion – electron method : The mass of an electron is 9.1 × 10 –31 kg. (iii) 2 moles of carbon are burnt in 16 g of dioxygen. (ii) 1 mole of carbon is burnt in 16 g of dioxygen. Hence, VSEPR theory also supports a tetrahedral structure for CH 4.Ĭalculate the amount of carbon dioxide that could be produced when Moreover, with a bond angle of 90° in square planar, the stability of CH 4 will be very less because of the repulsion existing between the bond pairs. Hence, the structure of CH 4 cannot be square planar. However, an atom of carbon does not have d-orbitals to undergo dsp 2 hybridization. Hence, carbon atom undergoes sp 3 hybridization in CH 4 molecule and takes a tetrahedral shape.įor a square planar shape, the hybridization of the central atom has to be dsp 2, ie, 1 s orbital, 3 p orbitals & 1 d orbitals has to undergo hybridization. Here 1 s orbital & 3 p orbitals under goes hybridization to form sp 3 hybrid orbitals. In the excited state, the orbital picture of carbon can be represented as: Now, Electronic configuration of carbon atom: The new orbitals formed are known as hybrid orbitals, it is called as hybridization. The phenomenon of mixing of orbitals of the same atom with slight difference in energies so as to redistribute their energies and give new orbitals of equivalent energy and shape.


 0 kommentar(er)
0 kommentar(er)
